Our Approach
Our proprietary, AI- and machine learning-guided discovery engine has yielded a portfolio of differentiated DNA editors capable of addressing more than 90% of the human genome.
Source From Nature
Employ sophisticated methods of curating metagenomic data
3 billion+ proteins indexed to date
AI/ML Optimization
High-throughput screening to identify and optimize editing technologies
AI/ML feedback loops used throughout discovery and optimization to improve speed/efficiency
Generate
New discoveries with novel properties
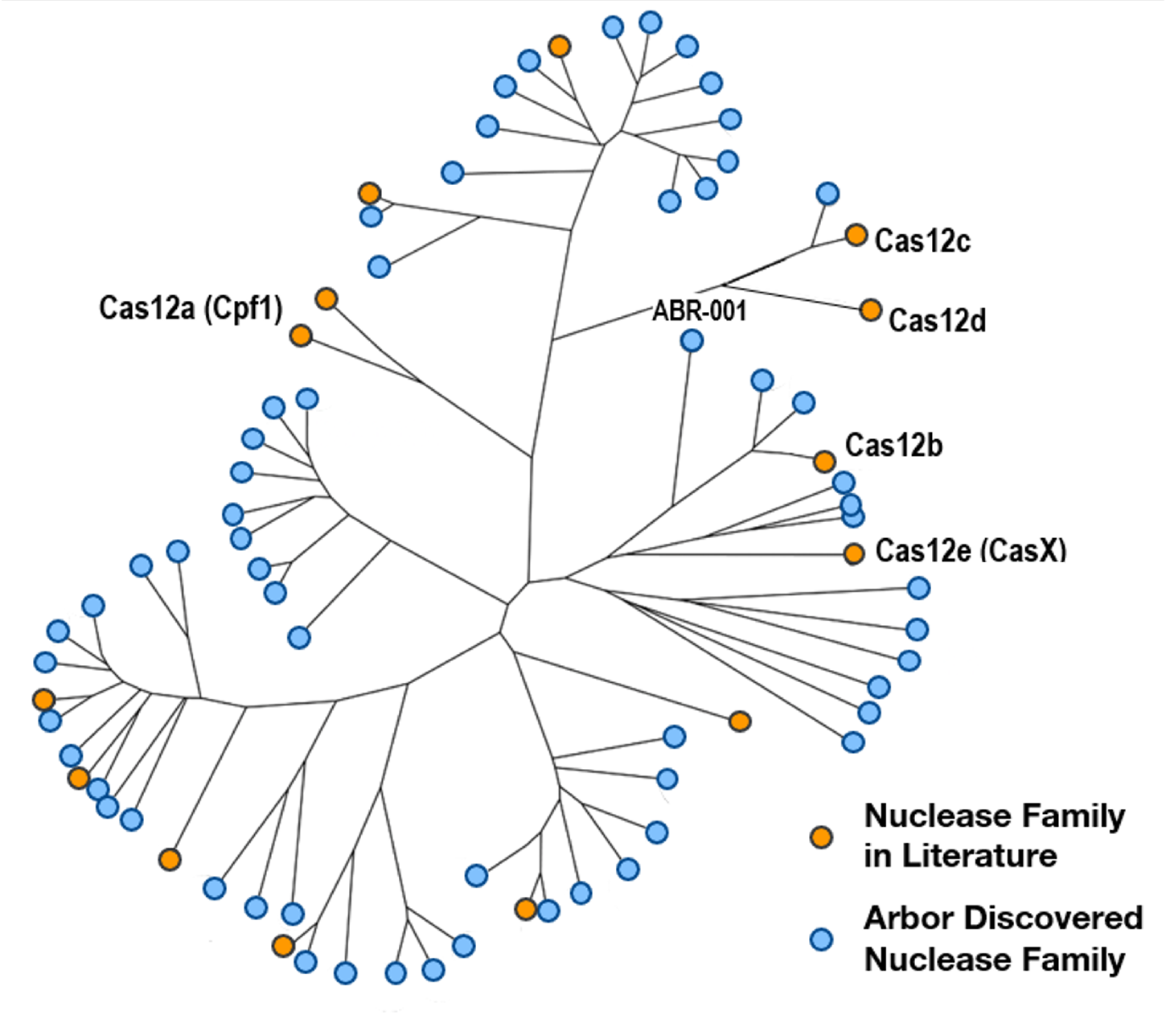
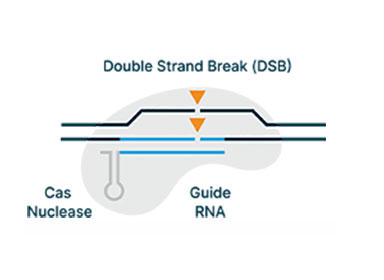
We have discovered 17 proprietary CRISPR subtypes and variants and 6x more nuclease families than are published in the literature
Engineering
Protein engineering to significantly improve editing efficiency

AI/ML driven engineering has generated >30x improvement in editing efficiency from the wild type nuclease to achieve therapeutically relevant levels of editing efficiency